Quantum Compliance adds critical updates to its Q-SDS proven software
Good software doesn’t remain static. Based on interaction with customers and real use cases, Quantum is continuously updating our software to provide more comprehensive solutions. Recently we implemented enhancements to our SDS software that save time and provide important international regional enhancements. Take a look.
The following updates are now implemented in the software and are active for all current customers. And of course, available for any user looking for a robust solution for their SDS authoring software needs.
Automatic PDF Output
Now a PDF of your SDS can be generated directly from the software. Rather than having to take the extra step of saving out to a WORD document first, the PDF is generated right from the HTML. Complete with headers, footers and page numbers, your final SDS is automatically generated making the process easier and saving you time.
International Updates
One of the key distinctives of Quantum’s offerings is that we have both a local and a global view of our customers’ needs. We are tuned into the details of every region, making certain that the nuances in the regulations and formats of that given region are built into our software. But we also are intentional in having a global reach in our offerings. It’s an expression of our commitment to think and act as a market leader on your behalf.
New/Updated GHS Output
We have recently updated templates for China, Australia, Mexico, Thailand, Indonesia and Europe. While many regions make use of the U.S.A. and European templates, we now offer the option to create GHS templates for other regions. These templates specifically reflect the different requirements and formats by region.
EU SDS Regulation Updates
If you sell chemicals in Europe, you are familiar with the Safety Data Sheet (SDS) regulation known as CLP. The CLP Regulation (for “Classification, Labelling and Packaging”) is a European Union regulation from 2008, which aligns the European Union system of classification, labelling and packaging of chemical substances and mixtures to the Globally Harmonized System (GHS).
CLP is part of the larger chemical regulation known as REACH (Regulation (EC) 2015/830). Major changes to the European SDS regulation became effective December 31, 2021. The EU SDS regulation Annex II to Regulation (EC) 2015/830 was replaced by the updated Regulation EU 2020/878. SDS complying with the 2015 regulation are no longer acceptable.
The update adopts revisions 6 and 7 of GHS and implements changes to many of the 16 sections of the SDS. We have completed a system wide GHS Classification review based on EU current CLP data and implemented these changes accordingly.
See here to learn more about the specific changes.
Multi-lingual SDS Output
In Canada versions of SDS in English and French are required. The same is true for labels. Which until this point meant two separate labels. However, we have now generated an advancement that includes English and French on the same label. This means significant cost and time savings in generation of the labels.
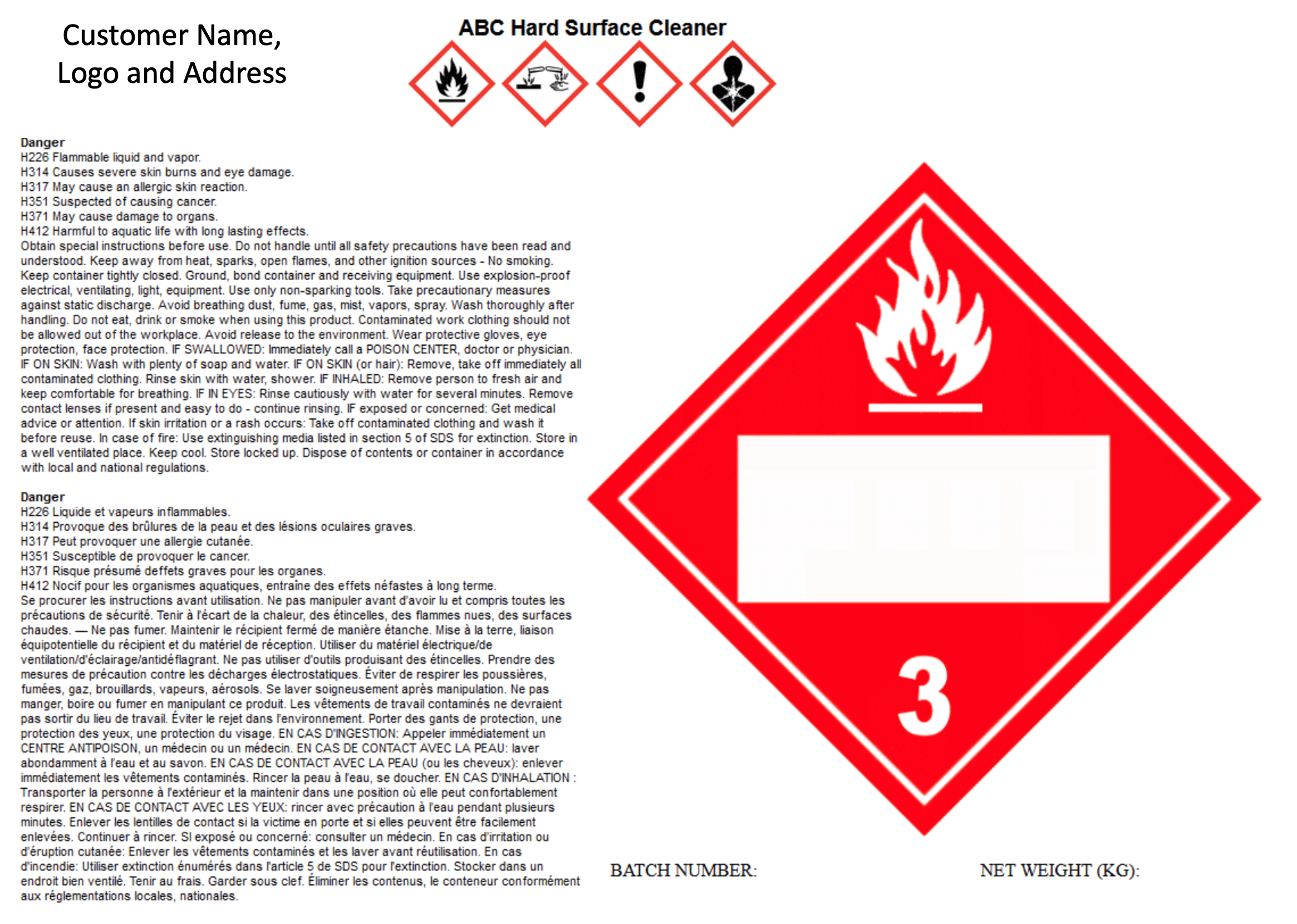
Country Specific Classifications
We now can offer the ability to create separate classifications for Japan and Korea using their country specific classifications. As a result, GHS classifications are now available in English, Japanese and Korean.
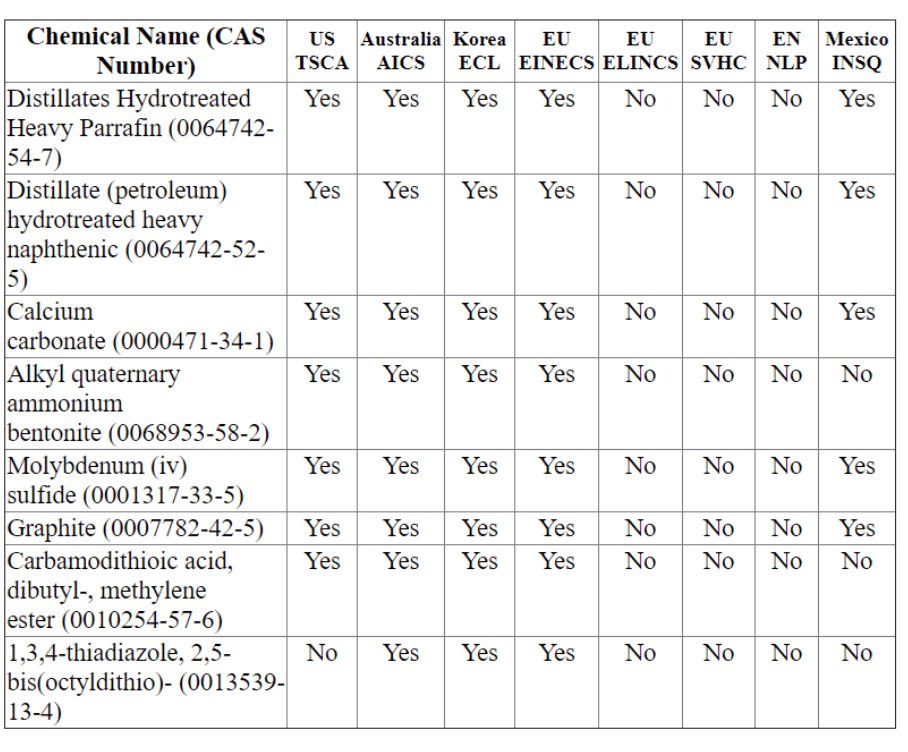
Section 15 World-Wide Lists of Lists
We have added the option to have a World-Wide List of Lists option. This allows any user the opportunity to access comparisons between regions.